Carbohydrates
Oh, those sweet carbs!
Many people can’t live without carbohydrates. The craving is understandable, because it has its roots in the very basis of biochemical processes of our bodies and functions in nature’s own living cycles.
All-mighty solar energy produces carbohydrates through photosynthesis and what is happening is that the inorganic carbon dioxide becomes organic carbon accompanied by the release of oxygen gas in the atmosphere. So, with the release of precious oxygen we get building element of carbs which are the most abundant biochemicals and are the raw materials for the synthesis of other biochemicals.

They are subdivided in three major groups:
- monosaccharides or simple sugars (3-9 carbon atoms in their structure),
- polysaccharides (polymers or compounds of simple ones) and
- oligosaccharides, intermediate group (2-10 carbons) which is maybe the most important group for humans because it is containing the subgroup called disaccharide which representative you may know as saccharose or sucrose amongst many others.
Keep it simple sweet
One of the most famous monosaccharides is D-glucose. D stands for dextrorotatory which means it rotates the plane of polarized light to the right – you may think this is too geeky and unimportant, but usability of glucose in your body depends on its optical feature, is it D or L form – does it rotate light to the right or left, cause L forms are not biologically important). D-glucose is famous for the reason because it is the building block of many other carbs.
It has 6 carbons as main structure and an ability to form the rings. For the sake of simplicity of presentation, in educational textbooks it is represented as planar and we used to draw it like that during our chemistry classes, but in reality it is not planar, it more like this…
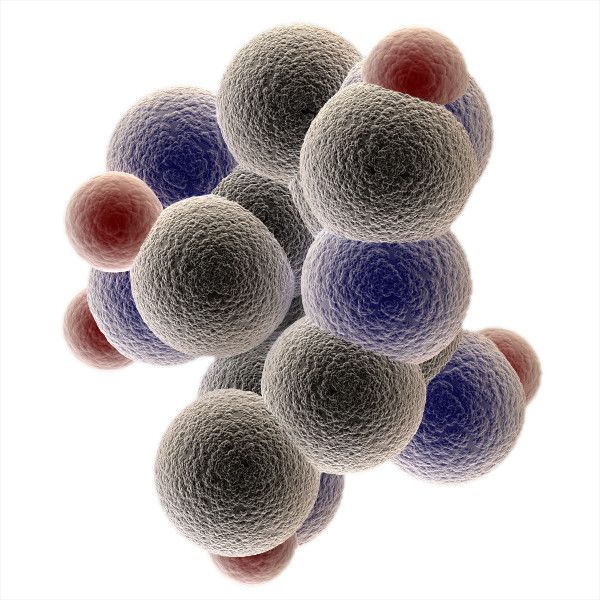
Glucose vs Fructose
Glucose is a six-member ring (pyranose structure), and fructose has the similar carbon formula, but it is a five-member ring (furanose structure) which is a more thermodynamically stable form. Glucose is also known as dextrose, and also as blood sugar, because it is commonly tested in blood if there is any suspicion on diabetes or prediabetic states.


Simple sugars form more complex structures by making glycosidic bonds or linkage, of which the simplest ones are disaccharides – the most famous one before-mentioned sucrose made of one glucose and one fructose.

What happens when we stack sugars together?
More complex structures are polysaccharides and you may know the representatives by the names starch and cellulose. Oh, breads, potatoes and pastas, those delicious starches! But pay attention to consume pastas and potatoes right after they are prepared because they form resistant starches that are almost indigestible when they are cooled down.
Polysaccharides are the polymers of D-glucose and they are the useful storage of it.
Starches appear in 3 forms: amylose, amylopectin and glycogen (which is an animal and human form of starch), they are very similar, different only in number and combination of glucose groups.


Cellulose is very similar to starch except there is a difference in the type of linkage between glucose structures which in the case of cellulose is beta 1-4. That’s one “tiny” feature that makes it indigestible for humans and that is the reason we can’t eat trees or woody plants (damn those lucky termites and cows!). Which is a shame, because cellulose is one of the most abundant biochemicals on this planet.

One major use of polysaccharides in our bodies is in the connective tissues like tendons, ligaments and collagen, whose structure contains hyaluronic acid for example, a disaccharide repeating unit in those important polysaccharides.